Introduction: Acute Myeloid Leukemia (AML) remains the most prevalent acute leukemia in adults, often associated with a bleak prognosis. Current treatment recommendations for elderly AML patients incorporate a combination of venetoclax (Ven) and azacitidine (AZA) following trials that underscored Ven's efficacy. This review seeks to analyze and summarize the literature concerning the effectiveness and safety of the Ven + AZA regimen for adult patients with newly diagnosed (ND) and relapsed/refractory (R/R) AML, and those unsuitable for intensive chemotherapy.
Materials and Methods: Following PRISMA guidelines, a comprehensive electronic literature search was conducted across PubMed, Embase, Clinicaltrials.gov, Cochrane Library, and Web of Science from inception to 2023. MeSH terms and relevant keywords for (Leukemia, Myeloid, Acute) AND (Venetoclax) AND (Azacitidine) were used. From 327 initially identified articles, 12 studies were selected for inclusion, and citation searching further added 2 studies, summing up to 14. We analyzed data from a combined patient pool of 1256.
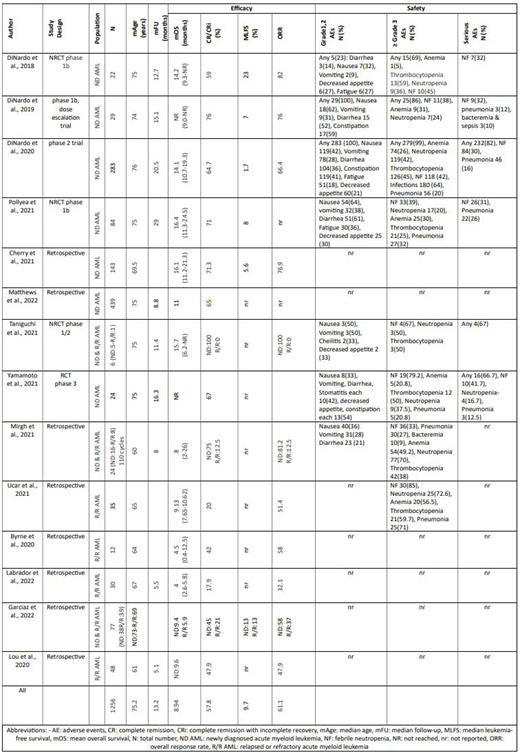
Results: The review encompassed 13 non-randomized and 1 randomized clinical trials. The median patient age was 75.2 years. Primary outcomes comprised complete remission (CR) and complete remission with incomplete count recovery (CRi), yielding a pooled CR/CRi of 57.8%. Secondary outcomes included an overall response rate (ORR) of 61.1%, and a median overall survival (mOS) of 8.94 months with a median follow-up duration of 13.25 months (Table 1). Notably, high-grade adverse effects were predominantly hematological (anemia, neutropenia, thrombocytopenia, febrile neutropenia) while low-grade effects primarily involved gastrointestinal symptoms (nausea, vomiting, diarrhea, constipation, decreased appetite, etc).
Conclusion: The current data suggest that the Ven + AZA regimen is both efficacious and well-tolerated for treating newly diagnosed and relapsed/refractory AML. Cytopenias across all three cell lines emerged as the most reported adverse effects of Ven + AZA therapy. Ongoing Phase III clinical trials are expected to enhance our understanding of this regimen's efficacy and safety profile, solidifying its role in standard AML therapy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal